Multiple Choice
In an experiment, 7.5 g of liquid water at 35 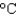 is converted into steam with a temperature of 145C. How much energy is required for this process?
is converted into steam with a temperature of 145C. How much energy is required for this process? 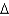 vap 2,260 J/g;
vap 2,260 J/g; 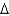 Hfus 334 J/g; cs(ice) 2.06 J/(g
Hfus 334 J/g; cs(ice) 2.06 J/(g 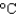 ) ; cs(water) 4.18 J/(g
) ; cs(water) 4.18 J/(g 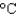 ) ; cs(steam) 1.99 J/(g
) ; cs(steam) 1.99 J/(g 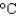 ) )
) )
A) 2.04 kJ
B) 17.0 kJ
C) 19.7 kJ
D) 5.40 kJ
E) 125 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q65: If 7.3 kJ of energy are required
Q66: The capacity to do work is a
Q67: A pot of water is heated with
Q68: Determine the enthalpy of formation for glucose
Q69: Use the following information to determine the
Q71: Describe the difference between potential energy and
Q72: Motor fuels are mixtures of hydrocarbons, but
Q73: Which statement regarding combustion of a sample
Q74: The basal metabolic rate (BMR) is the
Q75: The energy content of a Big Mac