Multiple Choice
Given the following thermochemical equation detailing the combustion of methane CH4(g) 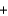 2O2(g)
2O2(g)  CO2(g)
CO2(g) 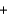 2H2O(g) Hrxn 802kJ/mol CH4
2H2O(g) Hrxn 802kJ/mol CH4
Determine the amount of energy released when 25.0 g of methane undergoes combustion.
A) 1.95 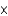
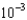 kJ
kJ
B) 3.12 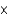
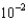 kJ
kJ
C) 453 kJ
D) 1250 kJ
E) 2.01 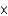
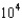 kJ
kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q20: State Hess's law as it applies to
Q21: Use the following information to determine the
Q22: For which reaction below does the enthalpy
Q23: Benzoic acid is used to determine the
Q24: Describe the difference between the change in
Q26: The integrated circuits in your cell phone
Q27: Explain what is meant by the term
Q28: Isooctane is a good model compound for
Q29: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q30: According to Coulomb's law, which ionic compound