Multiple Choice
For which reaction below does the enthalpy change under standard conditions correspond to a standard enthalpy of formation?
A) CO(g) 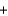 H2O(g)
H2O(g)  CO2(g)
CO2(g) 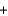 H2(g)
H2(g)
B) SO2(g) 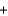
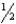 O2(g)
O2(g)  SO3(g)
SO3(g)
C) N2(g) 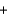 2O2(g)
2O2(g)  2NO2(g)
2NO2(g)
D) 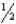 H2(g)
H2(g) 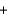
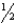 N2(g)
N2(g) 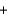
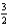 O2(g)
O2(g)  HNO3(g)
HNO3(g)
E) CH4(g) 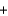 2O2(g)
2O2(g)  CO2(g)
CO2(g) 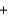 2H2O(g)
2H2O(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Work requires _<br>A)a use of potential energy.<br>B)a
Q18: What is the change in internal energy
Q19: Determine the enthalpy for the following reaction,
Q20: State Hess's law as it applies to
Q21: Use the following information to determine the
Q23: Benzoic acid is used to determine the
Q24: Describe the difference between the change in
Q25: Given the following thermochemical equation detailing the
Q26: The integrated circuits in your cell phone
Q27: Explain what is meant by the term