Multiple Choice
Use the following information to determine the enthalpy for the reaction shown below. CS2(l) 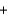 3O2(g)
3O2(g)  CO2(g)
CO2(g) 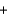 2SO2(g) H ?
2SO2(g) H ?
C(graphite) 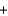 O2(g)
O2(g)  CO2(g) H 393.5 kJ
CO2(g) H 393.5 kJ
S(s) 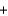 O2(g)
O2(g)  SO2(g) H 296.8 kJ
SO2(g) H 296.8 kJ
C(graphite) 2S(s)  CS2(l) H 87.9 kJ
CS2(l) H 87.9 kJ
A) (1075 kJ)
B) (1075 kJ)
C) (778 kJ)
D) (778 kJ)
E) (602 kJ)
Correct Answer:

Verified
Correct Answer:
Verified
Q16: A food sample was burned in a
Q17: Work requires _<br>A)a use of potential energy.<br>B)a
Q18: What is the change in internal energy
Q19: Determine the enthalpy for the following reaction,
Q20: State Hess's law as it applies to
Q22: For which reaction below does the enthalpy
Q23: Benzoic acid is used to determine the
Q24: Describe the difference between the change in
Q25: Given the following thermochemical equation detailing the
Q26: The integrated circuits in your cell phone