Short Answer
Use the following information to determine the enthalpy for the reaction shown below.
CS2(l) 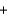 3O2(g)
3O2(g)  CO2(g)
CO2(g) 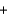 2SO2(g) Ho ?
2SO2(g) Ho ?
C(graphite) 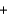 O2(g)
O2(g)  CO2(g) Ho 393.5 kJ
CO2(g) Ho 393.5 kJ
S(s) 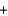 O2(g)
O2(g)  SO2(g) Ho 296.8 kJ
SO2(g) Ho 296.8 kJ
C(graphite) 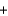 2S(s)
2S(s)  CS2(l) Ho 87.9 kJ
CS2(l) Ho 87.9 kJ
Correct Answer:

Verified
Ho F1...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q64: The best definition of the enthalpy change
Q65: If 7.3 kJ of energy are required
Q66: The capacity to do work is a
Q67: A pot of water is heated with
Q68: Determine the enthalpy of formation for glucose
Q70: In an experiment, 7.5 g of liquid
Q71: Describe the difference between potential energy and
Q72: Motor fuels are mixtures of hydrocarbons, but
Q73: Which statement regarding combustion of a sample
Q74: The basal metabolic rate (BMR) is the