Multiple Choice
Which one of the following is the molecular equation for the reaction of hydrobromic acid with potassium hydroxide? All species are in aqueous solution.
A) H+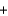 Br -
Br -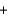 K+
K+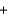 OH-
OH- KBr
KBr 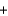 H2O
H2O
B) H+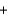 Br -
Br -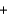 K+
K+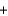 OH-
OH-  K+
K+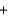 Br -
Br -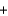 H2O
H2O
C) HBr 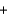 KOH
KOH  KBr
KBr 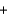 H2O
H2O
D) K+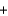 Br-
Br- KBr
KBr
E) H+ 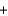 OH-
OH- H2O
H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q59: In a spontaneous oxidation reduction reaction between
Q60: A 125 mL sample of orange juice
Q61: The thermite reaction is used in welding
Q62: Dinitrogen monoxide (N<sub>2</sub>O) is produced from nitrate
Q63: Which of the following statements is not
Q65: In a titration, the solution of known
Q66: Some microorganisms living under anaerobic conditions extract
Q67: Which of the following compounds are soluble
Q68: Human blood contains about 0.18 g potassium
Q69: Sodium fluoride (NaF) is added to drinking