Multiple Choice
Identify the oxidizing agent in the following reaction. 2Hg22+(aq) 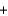 Sn2+(aq)
Sn2+(aq)  2Hg(l )
2Hg(l ) 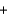 Sn4+(aq)
Sn4+(aq)
A) Hg(l)
B) Sn4+
C) Sn2+
D) Hg22+(aq)
E) This is not an example of an oxidation-reduction reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: A food chemist determined the concentration of
Q99: If there are 0.505 g of NaCl
Q100: Sodium metal easily loses an electron to
Q101: Magnesium hydroxide is insoluble. Write the net
Q102: Which of the following cartoons depicts a
Q104: Sodium thiosulfate (Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub>, molar mass = 158.2
Q105: Milk of Magnesia is a liquid antacid.
Q106: The carbon cycle characterizes the cyclic transformations
Q107: Tube worms that survive near geothermal vents
Q108: Write the net ionic equation for the