Multiple Choice
Sodium thiosulfate (Na2S2O3, molar mass = 158.2 g/mol) is used in the development of photographic film. Your summer job is at a photography lab and you need to check the purity of an outdated supply. You react 40.21 mL of 0.246 M iodine solution with a 3.232 g sample. What is the percent purity of the sodium thiosulfate that you report to your boss? I2(aq) 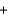 +2S2O32-(aq)
+2S2O32-(aq)  2I-(aq)
2I-(aq) 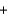 S4O62-(aq)
S4O62-(aq)
A) 100%
B) 48.4%
C) 96.8%
D) 98.6%
E) 84.4%
Correct Answer:

Verified
Correct Answer:
Verified
Q99: If there are 0.505 g of NaCl
Q100: Sodium metal easily loses an electron to
Q101: Magnesium hydroxide is insoluble. Write the net
Q102: Which of the following cartoons depicts a
Q103: Identify the oxidizing agent in the following
Q105: Milk of Magnesia is a liquid antacid.
Q106: The carbon cycle characterizes the cyclic transformations
Q107: Tube worms that survive near geothermal vents
Q108: Write the net ionic equation for the
Q109: The concentration of iron(II) ions in water