Multiple Choice
Write the net ionic equation for the reaction that takes place between a solution of potassium chloride and a solution of lead nitrate.
A) 2KCl(aq) 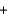 Pb(NO3) 2(aq)
Pb(NO3) 2(aq)  2KNO3(aq)
2KNO3(aq) 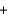 PbCl2(s)
PbCl2(s)
B) 2K+(aq) 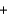 2Cl- (aq)
2Cl- (aq) 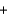 2Pb2+(aq)
2Pb2+(aq) 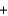 2NO3-(aq)
2NO3-(aq)  2KNO3(s)
2KNO3(s) 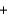 PbCl2(s)
PbCl2(s)
C) 2K+(aq) 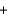 2NO3-(aq)
2NO3-(aq)  KNO3(aq)
KNO3(aq)
D) 2K+(aq) 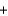 2Cl- (aq)
2Cl- (aq) 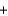 2Pb2+(aq)
2Pb2+(aq)  2NO3-(aq)
2NO3-(aq)  2K+(aq)
2K+(aq) 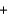 2NO3-(aq)
2NO3-(aq) 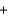 PbCl2(s)
PbCl2(s)
E) Pb2+(aq) 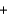 2Cl-(aq)
2Cl-(aq)  PbCl2(s)
PbCl2(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q103: Identify the oxidizing agent in the following
Q104: Sodium thiosulfate (Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub>, molar mass = 158.2
Q105: Milk of Magnesia is a liquid antacid.
Q106: The carbon cycle characterizes the cyclic transformations
Q107: Tube worms that survive near geothermal vents
Q109: The concentration of iron(II) ions in water
Q110: Write the balanced molecular, complete ionic, and
Q111: Write the complete ionic equation for the
Q112: In carrying out a titration of a
Q113: In its reaction with water, ammonia (NH<sub>3</sub>)