Multiple Choice
The concentration of iron(II) ions in water can be determined by titration with potassium permanganate in acid solution as shown by the incomplete unbalanced reaction equation below. Identify the stoichiometric coefficient of the iron(II) ion in the balanced reaction equation. Fe2+(aq) 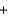 MnO4-
MnO4-
 Fe3+(aq)
Fe3+(aq) 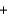 Mn2+(aq)
Mn2+(aq)
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Q104: Sodium thiosulfate (Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub>, molar mass = 158.2
Q105: Milk of Magnesia is a liquid antacid.
Q106: The carbon cycle characterizes the cyclic transformations
Q107: Tube worms that survive near geothermal vents
Q108: Write the net ionic equation for the
Q110: Write the balanced molecular, complete ionic, and
Q111: Write the complete ionic equation for the
Q112: In carrying out a titration of a
Q113: In its reaction with water, ammonia (NH<sub>3</sub>)
Q114: The World Health Organization recommends that the