Multiple Choice
In one analysis, the density of ozone in an air sample was found to be 5.00 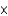 10-8 mol/L. What is the density in molecules per liter?
10-8 mol/L. What is the density in molecules per liter?
A) 8.30 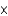 10-32
10-32
B) 3.01 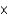 1016
1016
C) 1.81 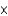 10-11
10-11
D) 3.01 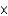 1011
1011
E) 6.02 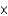 1023
1023
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Which statement about the following chemical
Q61: The combustion of ethanol (CH<sub>3</sub>CH<sub>2</sub>OH, 46.1 g/mol)
Q62: Which statement about a balanced chemical reaction
Q63: A compound was analyzed and found to
Q64: Baking soda (NaHCO<sub>3</sub>, 84.0 g/mol) requires acids
Q66: Write the balanced reaction equation for the
Q67: Diborane undergoes combustion according to the following
Q68: Which statement about the following chemical reaction
Q69: The most abundant metal in Earth's crust
Q70: A gallon of water has a mass