Essay
Diborane undergoes combustion according to the following unbalanced reaction.
B2H6 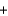 O2
O2  HBO2
HBO2 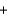 H2O
H2O
Balance the equation and calculate how much HBO2 can be formed from 12.6 g of B2H6?
Correct Answer:

Verified
B2H6  3O...
3O...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q62: Which statement about a balanced chemical reaction
Q63: A compound was analyzed and found to
Q64: Baking soda (NaHCO<sub>3</sub>, 84.0 g/mol) requires acids
Q65: In one analysis, the density of ozone
Q66: Write the balanced reaction equation for the
Q68: Which statement about the following chemical reaction
Q69: The most abundant metal in Earth's crust
Q70: A gallon of water has a mass
Q71: Household bleach contains 5.0% sodium hypochlorite (NaOCl)
Q72: A hydrocarbon molecule contains carbon and hydrogen