Multiple Choice
Baking soda (NaHCO3, 84.0 g/mol) requires acids from other ingredients to generate the carbon dioxide needed to make bread rise. The following equation describes this reaction, where HB is some unspecified acid. If 20.4 g of baking soda are used in a recipe and enough acid is present for a complete reaction, how many moles of carbon dioxide are generated? HB 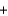 NaHCO3
NaHCO3  H2O
H2O 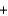 CO2
CO2 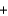 NaB
NaB
A) 0.464 mol
B) 0.334 mol
C) 0.243 mol
D) 0.204 mol
E) 0.232 mol
Correct Answer:

Verified
Correct Answer:
Verified
Q59: As a purchasing agent for a pharmaceutical
Q60: Which statement about the following chemical
Q61: The combustion of ethanol (CH<sub>3</sub>CH<sub>2</sub>OH, 46.1 g/mol)
Q62: Which statement about a balanced chemical reaction
Q63: A compound was analyzed and found to
Q65: In one analysis, the density of ozone
Q66: Write the balanced reaction equation for the
Q67: Diborane undergoes combustion according to the following
Q68: Which statement about the following chemical reaction
Q69: The most abundant metal in Earth's crust