Multiple Choice
Phosphorus trichloride reacts with water to form phosphorous acid, H3PO3, and hydrochloric acid in the following unbalanced reaction. For each mole of phosphorous acid produced by this reaction, how many moles of HCl are produced?__ PCl3 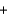 __H2O
__H2O  __H3PO3
__H3PO3 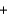 + __ HCl
+ __ HCl
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Q120: The combustion of fossil fuels is the
Q121: What is the molar mass of sulfuric
Q122: The empirical formula for buckminsterfullerene is C<sub>1</sub>,
Q123: Caffeine has an elemental analysis of 49.48%
Q124: How many nitrogen atoms are there in
Q126: Identify the list below that has these
Q127: Mesitylene is a liquid hydrocarbon. If 0.115
Q128: The combustion of heptane (C<sub>7</sub>H<sub>16</sub>) forms carbon
Q129: A copper ore consisting of 12.5% copper(II)
Q130: A range of organic molecules can undergo