Multiple Choice
Identify the list below that has these chloride compounds arranged in order of increasing molar mass.
A) CCl4 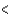 PCl3
PCl3
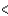 SCl2
SCl2 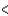 BCl3
BCl3
B) SCl2 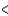 BCl3
BCl3 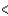 PCl3
PCl3 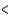 CCl4
CCl4
C) BCl3 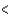 CCl4
CCl4 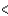 PCl3
PCl3 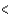 SCl2
SCl2
D) SCl2 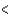 BCl3
BCl3 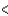 CCl4
CCl4 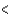 PCl3
PCl3
E) BCl3 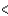 SCl2
SCl2 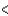 PCl3
PCl3 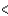 CCl4
CCl4
Correct Answer:

Verified
Correct Answer:
Verified
Q121: What is the molar mass of sulfuric
Q122: The empirical formula for buckminsterfullerene is C<sub>1</sub>,
Q123: Caffeine has an elemental analysis of 49.48%
Q124: How many nitrogen atoms are there in
Q125: Phosphorus trichloride reacts with water to form
Q127: Mesitylene is a liquid hydrocarbon. If 0.115
Q128: The combustion of heptane (C<sub>7</sub>H<sub>16</sub>) forms carbon
Q129: A copper ore consisting of 12.5% copper(II)
Q130: A range of organic molecules can undergo
Q131: Metallic sodium was originally produced by