Multiple Choice
The combustion of heptane (C7H16) forms carbon dioxide and water. What is the stoichiometric coefficient for water in the balanced equation when 1 mol of heptane undergoes combustion?
A) 6
B) 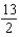
C) 7
D) 8
E) 9
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q123: Caffeine has an elemental analysis of 49.48%
Q124: How many nitrogen atoms are there in
Q125: Phosphorus trichloride reacts with water to form
Q126: Identify the list below that has these
Q127: Mesitylene is a liquid hydrocarbon. If 0.115
Q129: A copper ore consisting of 12.5% copper(II)
Q130: A range of organic molecules can undergo
Q131: Metallic sodium was originally produced by
Q132: Write a definition of the term molar
Q133: In which of the following reaction mixtures,