Multiple Choice
A fuel cell works on the same principle as a battery but is continually fed with fuel. In one fuel cell, methanol (CH3OH) enters on one side of the unit and air enters on the other side. Both circulate past electrodes, and a chemical reaction occurs that produces electricity, plus carbon dioxide and water as byproducts. Which balanced chemical reaction equation best represents the reaction that occurs in this fuel cell?
A) CH3OH  CO2
CO2 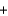 H2O
H2O
B) CH3OH  CH3
CH3 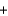 OH
OH
C) CH3OH  CH2
CH2 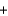 H2O
H2O
D) CH3OH 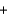 O2
O2  CO2
CO2 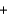 H2O
H2O
E) 2CH3OH 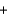 3O2
3O2  2CO2
2CO2 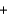 4H2O
4H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q68: Which statement about the following chemical reaction
Q69: The most abundant metal in Earth's crust
Q70: A gallon of water has a mass
Q71: Household bleach contains 5.0% sodium hypochlorite (NaOCl)
Q72: A hydrocarbon molecule contains carbon and hydrogen
Q74: Why is the term formula unit more
Q75: Metallic sodium was originally produced by
Q76: The average adult exhales about 1.0 kg
Q77: Which statement A-D regarding the carbon cycle
Q78: What are the stoichiometric coefficients for oxygen