Multiple Choice
Metallic sodium was originally produced by the Deville process, where sodium carbonate and carbon black are heated to 1100 C to produce sodium (as a liquid at that temperature) and carbon monoxide gas, according to the following balanced equation. Na2CO3(s) 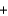 2C(s)
2C(s)  2Na(l)
2Na(l) 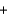 3CO(g)
3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
A) 0.50 kg
B) 1.0 kg
C) 1.5 kg
D) 11 kg
E) 21 kg
Correct Answer:

Verified
Correct Answer:
Verified
Q70: A gallon of water has a mass
Q71: Household bleach contains 5.0% sodium hypochlorite (NaOCl)
Q72: A hydrocarbon molecule contains carbon and hydrogen
Q73: A fuel cell works on the same
Q74: Why is the term formula unit more
Q76: The average adult exhales about 1.0 kg
Q77: Which statement A-D regarding the carbon cycle
Q78: What are the stoichiometric coefficients for oxygen
Q79: Baking ammonia or ammonium bicarbonate (NH<sub>4</sub>HCO<sub>3</sub>, 79.1
Q80: Hydrogen peroxide decomposes to produce water