Multiple Choice
Household bleach contains 5.0% sodium hypochlorite (NaOCl) by mass. Household ammonia contains 5.0% NH3 by mass. If 50.00 g of bleach are mixed with 50.00 g of 5.0% ammonia, what mass of the toxic chloramine gas would be produced by the following reaction? NaOCl 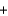 NH3
NH3  NH2Cl
NH2Cl 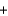 NaOH
NaOH
A) 5.0 g
B) 2.5 g
C) 1.7 g
D) 7.6 g
E) 3.2 g
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Write the balanced reaction equation for the
Q67: Diborane undergoes combustion according to the following
Q68: Which statement about the following chemical reaction
Q69: The most abundant metal in Earth's crust
Q70: A gallon of water has a mass
Q72: A hydrocarbon molecule contains carbon and hydrogen
Q73: A fuel cell works on the same
Q74: Why is the term formula unit more
Q75: Metallic sodium was originally produced by
Q76: The average adult exhales about 1.0 kg