Multiple Choice
Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced? 2H2O(l)  2H2(g)
2H2(g) 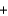 O2(g)
O2(g)
A) 17.8 g
B) 8.9 g
C) 35.6 g
D) 16.0 g
E) 10.0 g
Correct Answer:

Verified
Correct Answer:
Verified
Q90: Which statement about the combustion of propane
Q91: Glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) is oxidized by molecular oxygen
Q92: Calcium hydride (CaH<sub>2</sub>) is so reactive with
Q93: A mass of 11.60 g of phosphoric
Q94: Sulfur dioxide from coal-fired power plants combines
Q96: The acid-base reaction between phosphoric acid, H<sub>3</sub>PO<sub>4</sub>,
Q97: Beryllium metal reacts with chlorine gas to
Q98: U.S. Lime & Minerals is a company
Q99: Fool's Gold is the mineral pyrite (FeS<sub>2</sub>).
Q100: Which one of the following samples contains