Multiple Choice
Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is: CaH2(s) 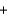 2H2O(l)
2H2O(l)  Ca(OH) 2(s)
Ca(OH) 2(s) 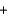 2H2(g)
2H2(g)
A) CaH2 will be left over.
B) Water will be left over.
C) Both will be completely consumed.
D) There is no way to tell.
Correct Answer:

Verified
Correct Answer:
Verified
Q87: Some indoor air purification systems work by
Q88: You are given a liquid sample that
Q89: For a standard airbag, 131 g of
Q90: Which statement about the combustion of propane
Q91: Glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) is oxidized by molecular oxygen
Q93: A mass of 11.60 g of phosphoric
Q94: Sulfur dioxide from coal-fired power plants combines
Q95: Water can be separated into its elements
Q96: The acid-base reaction between phosphoric acid, H<sub>3</sub>PO<sub>4</sub>,
Q97: Beryllium metal reacts with chlorine gas to