Multiple Choice
Arizona was the site of a 400,000-acre wildfire in June 2002. How much carbon dioxide was produced by this fire? Assume that the density of carbon on the acreage was 10 kg/m2 and that 50% of the biomass burned. (10,000 m2 = 2.47 acre)
A) 3 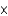 1010 kg
1010 kg
B) 3 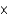 106 kg
106 kg
C) 3 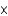 1030 kg
1030 kg
D) 3 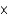 109 kg
109 kg
E) 3 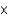 1013 kg
1013 kg
Correct Answer:

Verified
Correct Answer:
Verified
Q2: How many mL of hexadecane (C<sub>16</sub>H<sub>34</sub>) are
Q3: What mass of phosphoric acid (H<sub>3</sub>PO<sub>4</sub>, 98.00
Q4: One reaction that occurs during the deployment
Q5: If the combustion of fossil fuels
Q6: A range of organic molecules can undergo
Q8: Burning coal that contains sulfur releases sulfur
Q9: In a self-contained breathing apparatus, potassium superoxide,
Q10: Methylheptenone (MHP) is used in producing lemon
Q11: An iron ore, magnetite, contains only iron
Q12: In one analysis, 3.01 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In