Multiple Choice
One reaction that occurs during the deployment of an air bag is shown below, where sodium metal reacts with potassium nitrate. If 5.10 g of sodium and 305 g of potassium nitrate react in an airbag, how many grams of KNO3 remain because of the limited amount of sodium present? 10Na(s) 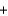 2KNO3(s)
2KNO3(s)  K2O(s)
K2O(s) 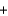 5Na2O(s)
5Na2O(s) 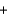 N2(g)
N2(g)
A) 2.24 g
B) 112 g
C) 193 g
D) 301 g
E) 303 g
Correct Answer:

Verified
Correct Answer:
Verified
Q1: In a demonstration of the complete combustion
Q2: How many mL of hexadecane (C<sub>16</sub>H<sub>34</sub>) are
Q3: What mass of phosphoric acid (H<sub>3</sub>PO<sub>4</sub>, 98.00
Q5: If the combustion of fossil fuels
Q6: A range of organic molecules can undergo
Q7: Arizona was the site of a 400,000-acre
Q8: Burning coal that contains sulfur releases sulfur
Q9: In a self-contained breathing apparatus, potassium superoxide,
Q10: Methylheptenone (MHP) is used in producing lemon
Q11: An iron ore, magnetite, contains only iron