Multiple Choice
In a self-contained breathing apparatus, potassium superoxide, KO2, reacts with carbon dioxide to form potassium carbonate and oxygen. Identify the limiting reactant and determine the amount of oxygen gas, in moles, that can be produced from 250 g of KO2 and 450 g of CO2. 4KO2(s) 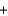 2CO2(g)
2CO2(g)  2K2CO3(s)
2K2CO3(s) 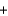 3O2(g)
3O2(g)
A) potassium superoxide limiting, 4.75 mol
B) potassium superoxide limiting, 1.53 mol
C) potassium superoxide limiting, 2.64 mol
D) carbon dioxide limiting, 1.53 mol
E) carbon dioxide limiting, 2.64 mol
Correct Answer:

Verified
Correct Answer:
Verified
Q4: One reaction that occurs during the deployment
Q5: If the combustion of fossil fuels
Q6: A range of organic molecules can undergo
Q7: Arizona was the site of a 400,000-acre
Q8: Burning coal that contains sulfur releases sulfur
Q10: Methylheptenone (MHP) is used in producing lemon
Q11: An iron ore, magnetite, contains only iron
Q12: In one analysis, 3.01 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q13: Acetaminophen is a common medication used to
Q14: Azobenzene is a highly colored organic compound.