Multiple Choice
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The emergency spill kit contained a full 2.0 kg bottle of sodium carbonate (105.99 g/mol) . Is this enough sodium carbonate to neutralize the acid, according to the following reaction? H2SO4(aq) 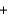 Na2CO3(s)
Na2CO3(s)  Na2SO4(aq)
Na2SO4(aq) 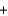 CO2(g)
CO2(g) 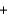 H2O(l)
H2O(l)
A) Yes, there is about 50% more than what is needed.
B) Yes, there is exactly enough sodium carbonate, but no excess.
C) No, there is not enough sodium carbonate, but the amount is only about 10% too small.
D) No, there is not nearly enough sodium carbonate.
E) Yes, but there is only about 10% excess.
Correct Answer:

Verified
Correct Answer:
Verified
Q80: Hydrogen peroxide decomposes to produce water
Q81: What is the molar mass of phosphorus
Q82: One form of elemental sulfur is a
Q83: A reaction vessel contains equal masses of
Q84: How many atoms of chlorine are there
Q86: Write the balanced reaction equation for the
Q87: Some indoor air purification systems work by
Q88: You are given a liquid sample that
Q89: For a standard airbag, 131 g of
Q90: Which statement about the combustion of propane