Hydrogen Peroxide Decomposes to Produce Water and Oxygen 2H2O + O2
A)2 Molecules 2 Molecules +1 Molecules
Multiple Choice
Hydrogen peroxide decomposes to produce water and oxygen. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2H2O2 2H2O + O2
A) 2 molecules  2 molecules +1 molecules
2 molecules +1 molecules
B) 34.0 g  18.0 g
18.0 g 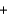 16.0 g
16.0 g
C) 68.0 g  36.0 g
36.0 g 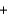 32.0 g
32.0 g
D) 2x mol  2x mol
2x mol 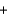 x mol
x mol
E) y(34.0 g)  y(18.0 g)
y(18.0 g) 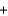 y(32 g)
y(32 g)
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Metallic sodium was originally produced by
Q76: The average adult exhales about 1.0 kg
Q77: Which statement A-D regarding the carbon cycle
Q78: What are the stoichiometric coefficients for oxygen
Q79: Baking ammonia or ammonium bicarbonate (NH<sub>4</sub>HCO<sub>3</sub>, 79.1
Q81: What is the molar mass of phosphorus
Q82: One form of elemental sulfur is a
Q83: A reaction vessel contains equal masses of
Q84: How many atoms of chlorine are there
Q85: A bottle containing 1,665 g of sulfuric