Multiple Choice
A reaction vessel contains equal masses of solid magnesium metal and oxygen gas. The mixture is ignited and burns with a burst of light and heat, producing solid MgO. The mass of the MgO is less than the initial mass of the magnesium and oxygen. What is your explanation for this apparent loss of mass?
A) Conservation of mass is violated in this reaction.
B) Some of the mass was converted into energy (heat and light) as 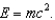
C) Not all of the oxygen reacted.
D) Not all of the magnesium reacted.
E) Measurement must be in error because mass is conserved in chemical reactions.
Correct Answer:

Verified
Correct Answer:
Verified
Q78: What are the stoichiometric coefficients for oxygen
Q79: Baking ammonia or ammonium bicarbonate (NH<sub>4</sub>HCO<sub>3</sub>, 79.1
Q80: Hydrogen peroxide decomposes to produce water
Q81: What is the molar mass of phosphorus
Q82: One form of elemental sulfur is a
Q84: How many atoms of chlorine are there
Q85: A bottle containing 1,665 g of sulfuric
Q86: Write the balanced reaction equation for the
Q87: Some indoor air purification systems work by
Q88: You are given a liquid sample that