Short Answer
Metallic sodium was originally produced by the Deville process, where sodium carbonate and carbon black are heated to 1100 C to produce sodium (as a liquid at that temperature) and carbon monoxide gas, according to the following balanced equation.
Na2CO3(s ) 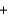 2C(s )
2C(s )  2Na(l)
2Na(l) 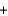 3CO(g)
3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
Correct Answer:

Verified
Correct Answer:
Verified
Q126: Identify the list below that has these
Q127: Mesitylene is a liquid hydrocarbon. If 0.115
Q128: The combustion of heptane (C<sub>7</sub>H<sub>16</sub>) forms carbon
Q129: A copper ore consisting of 12.5% copper(II)
Q130: A range of organic molecules can undergo
Q132: Write a definition of the term molar
Q133: In which of the following reaction mixtures,
Q134: Which of the following cartoons depict a
Q135: What is the molar mass of glucose,
Q136: Copper was the first metal to be