Multiple Choice
If a reaction A B has G 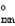 > 0, then when the reaction is at equilibrium there will be __________
> 0, then when the reaction is at equilibrium there will be __________
A) absolutely no B that is formed.
B) smaller quantities of B and larger quantities of A.
C) equal quantities of A and B.
D) large quantities of B and smaller quantities of A.
E) absolutely no A remaining.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: In a spontaneous process, the entropy of
Q28: A reaction is at equilibrium at a
Q32: Which of the following processes will lead
Q33: In a spontaneous process, which of the
Q35: "<font face="symbol"></font>S<sub>sys</sub>" can be directly related to
Q36: Determine the normal melting point of benzoic
Q41: The gas above the liquid in
Q53: At 0 K, the entropy of a
Q64: Dinitrogen tetroxide (N<sub>2</sub>O<sub>4</sub>) decomposes to nitrogen
Q79: The entropy of vaporization of water is