Multiple Choice
The Lewis structure of formaldehyde is shown below. Which statement concerning this structure is incorrect? 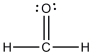
A) Two electrons are being shared between the carbon atom and the oxygen atom.
B) The oxygen atom has four valence electrons that are not being shared with another atom.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have filled valence shells.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A double bond consists of four electrons
Q35: Some covalent compounds are solids,some are liquids,and
Q54: Rank the atoms Br,Cl,and K in order
Q73: Which atom(s)in the structure below has(have)a
Q76: Aspartic acid is an amino acid used
Q78: The Lewis structure for the molecule below
Q79: What is the molecular shape around the
Q80: The molecule below is a polar molecule.
Q81: The covalent bond between chlorine and
Q82: How many lone pairs of electrons need