Multiple Choice
For the reaction 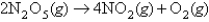 ,the following data were collected:
,the following data were collected: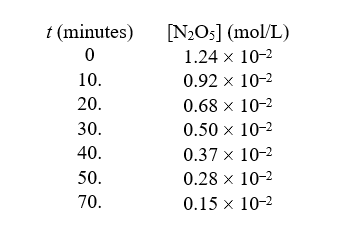
-The order of this reaction in N2O5 is
A) 0
B) 1
C) 2
D) 3
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: The reaction A <span class="ql-formula"
Q62: The initial rate of production of
Q63: Two mechanisms are proposed: I. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q64: What is the numerical value of the
Q65: For the second-order reaction NO(g)+ O<sub>3</sub>(g)
Q67: The rate is constant over time.
Q68: Consider the following rate law: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q69: The elementary chemical reaction O +
Q70: The rate constant k is dependent on<br>I.the
Q71: Consider the reaction 3A + B