Multiple Choice
The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 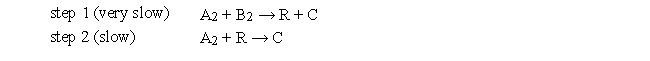
-The rate constant for a reaction increases from 10.0 s-1 to 100. s-1 when the temperature is increased from 315K to 416K. What is the activation energy for the reaction in kJ/mol? (R = 8.314 J/mol • K)
A) 10.8 kJ/mol
B) 0.0823 kJ/mol
C) 1.90 kJ/mol
D) 19.3 kJ/mol
E) 24.8 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q61: The hypothetical reaction<br>2A → 2B + D<br>is
Q62: The following questions refer to the reaction
Q63: The following questions refer to the hypothetical
Q64: For the reaction aA → products, select
Q65: For the reaction<br>2NO(g) + H<sub>2</sub>(g) → N<sub>2</sub>O(g)
Q67: For a reaction a A → products,
Q68: For the reaction<br>2A + B → products<br>the
Q69: The following question refers to the gas-phase
Q70: The reaction<br>H<sub>2</sub>SeO<sub>3</sub>(aq) + 6I<sup>-</sup>(aq) + 4H<sup>+</sup>(aq) →
Q71: Two isomers (A and B) of a