Short Answer
The hypothetical reaction
2A → 2B + D
is assumed to have the mechanism 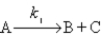
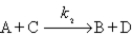
Derive the differential rate law for the production of D using the steady-state approximation.
Correct Answer:

Verified
Correct Answer:
Verified
Q56: For the reaction<br>CH<sub>3</sub>CHCH<sub>2</sub>(g) + HCl(g) → CH<sub>3</sub>CHClCH<sub>3</sub>(g)<br>a
Q57: The reaction<br>3NO → N<sub>2</sub>O + NO<sub>2</sub><br>Is found
Q58: At a particular temperature, N<sub>2</sub>O<sub>5</sub> decomposes according
Q59: Use the potential energy diagram shown to
Q60: In the reaction<br>3A(g) + B(g) → 2C(g)
Q62: The following questions refer to the reaction
Q63: The following questions refer to the hypothetical
Q64: For the reaction aA → products, select
Q65: For the reaction<br>2NO(g) + H<sub>2</sub>(g) → N<sub>2</sub>O(g)
Q66: The following questions refer to the reaction