Short Answer
For the reaction
2NO(g) + H2(g) → N2O(g) + H2O(g)
two mechanisms, A and B, have been proposed. Derive the rate law for each proposed mechanism for the production of N2O. 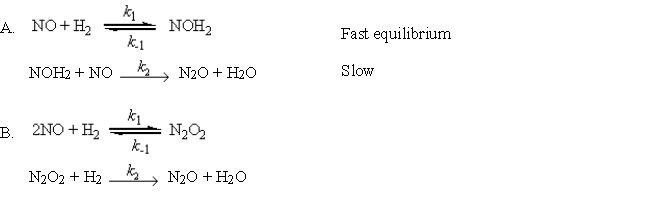
For mechanism B, use the steady-state approximation. Let rate 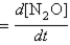
Correct Answer:

Verified
A. Rate = ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q60: In the reaction<br>3A(g) + B(g) → 2C(g)
Q61: The hypothetical reaction<br>2A → 2B + D<br>is
Q62: The following questions refer to the reaction
Q63: The following questions refer to the hypothetical
Q64: For the reaction aA → products, select
Q66: The following questions refer to the reaction
Q67: For a reaction a A → products,
Q68: For the reaction<br>2A + B → products<br>the
Q69: The following question refers to the gas-phase
Q70: The reaction<br>H<sub>2</sub>SeO<sub>3</sub>(aq) + 6I<sup>-</sup>(aq) + 4H<sup>+</sup>(aq) →