Multiple Choice
Consider the following table of relative strengths of oxidizing and reducing agents:
Oxidizing Agent
Reducing Agent
Stronger
A+ + e- 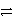
A
Weaker
B2+ + 2 e- 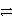
B
Weaker
C3+ + 3 e- 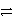
C
Stronger
Which correctly lists the oxidizing agents in order of increasing strength?
A) A < B < C
B) C < B < A
C) A+ < B2+ < C3+
D) B2+ < A+ < C3+
E) C3+ < B2+ < A+
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which among the following are the parts
Q7: Consider the following beakers.In the beaker on
Q8: What is the reducing agent in the
Q10: What is the oxidation number of sulfur
Q12: What is the oxidation number of titanium
Q12: Which of the following is the half-reaction
Q13: Examine the two beakers shown below. <img
Q14: Which substance gets reduced in this redox
Q15: Consider the following table of relative
Q16: How many electrons are transferred in the