Multiple Choice
Consider the following beakers.In the beaker on the left copper metal is placed in a solution of silver nitrate.The reaction is allowed to run for 60 minutes producing the products shown on the right. 

The equation for the oxidation half-reaction would be:
A) 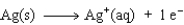
B) 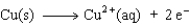
C) 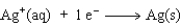
D) 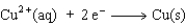
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Identify the reduction half-reaction in the redox
Q4: In the reaction of carbon with oxygen,which
Q5: What is the oxidizing agent in this
Q6: Which among the following are the parts
Q8: What is the reducing agent in the
Q10: What is the oxidation number of sulfur
Q11: Consider the following table of relative
Q12: What is the oxidation number of titanium
Q12: Which of the following is the half-reaction
Q41: When zinc is plated to iron,the zinc