Multiple Choice
What is the reducing agent in the reaction of hydrazine as a rocket fuel: N2H4( 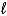 ) + O2(g)
) + O2(g) 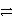 N2(g) + 2 H2O(
N2(g) + 2 H2O( 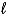 ) ?
) ?
A) N2H4
B) O2
C) N2
D) H2O
E) There is no reducing agent in this reaction
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Identify the reduction half-reaction in the redox
Q4: In the reaction of carbon with oxygen,which
Q5: What is the oxidizing agent in this
Q6: Which among the following are the parts
Q7: Consider the following beakers.In the beaker on
Q10: What is the oxidation number of sulfur
Q11: Consider the following table of relative
Q12: What is the oxidation number of titanium
Q12: Which of the following is the half-reaction
Q13: Examine the two beakers shown below. <img