Multiple Choice
In an experiment,5.585 g of iron metal reacts with 3.207 g of yellow sulfur.Using the conservation of mass law,predict the mass of product. Fe(s) + S(s) 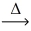 FeS(s)
FeS(s)
A) 2.198 g
B) 2.378 g
C) 4.396 g
D) 8.792 g
E) 17.584 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Considering the limiting reactant,what is the volume
Q102: How many moles of oxygen gas react
Q103: How many moles of hydrogen gas react
Q104: What is the mass of sodium nitrate
Q105: What is the term for the amount
Q107: Considering the limiting reactant concept,how many moles
Q108: How many moles of chlorine gas react
Q109: How many moles of hydrogen gas are
Q110: How many moles of nitrogen dioxide gas
Q111: What volume of methane gas,CH₄,reacts to give