Multiple Choice
How many moles of oxygen gas react to yield 0.100 mol water? __C5H₁₂ (g) + __O₂(g) 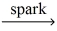 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
A) 0.100 mol
B) 0.500 mol
C) 0.600 mol
D) 0.800 mol
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q97: Considering the limiting reactant,what is the volume
Q98: Starting with 1.56 g of salicylic acid,a
Q99: Why was Haber denounced by American,English,and French
Q100: What is the term for the relationship
Q101: Considering the limiting reactant,what is the volume
Q103: How many moles of hydrogen gas react
Q104: What is the mass of sodium nitrate
Q105: What is the term for the amount
Q106: In an experiment,5.585 g of iron metal
Q107: Considering the limiting reactant concept,how many moles