Multiple Choice
What is the mass of sodium nitrate (85.00 g/mol) that decomposes to release 2.55 L of oxygen gas at STP? __NaNO₃(s) 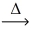 __NaNO₂(s) + __O₂(g)
__NaNO₂(s) + __O₂(g)
A) 0.336 g
B) 1.34 g
C) 4.84 g
D) 9.68 g
E) 19.4 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q99: Why was Haber denounced by American,English,and French
Q100: What is the term for the relationship
Q101: Considering the limiting reactant,what is the volume
Q102: How many moles of oxygen gas react
Q103: How many moles of hydrogen gas react
Q105: What is the term for the amount
Q106: In an experiment,5.585 g of iron metal
Q107: Considering the limiting reactant concept,how many moles
Q108: How many moles of chlorine gas react
Q109: How many moles of hydrogen gas are