Essay
Write an expression for the equilibrium constant (Keq) for the following reaction. 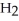 (g) +
(g) + 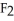 (g)
(g)  2HF (g)
2HF (g)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q116: The concentrations of reactants and products do
Q117: Consider the reaction with Kc = 0.211.
Q118: A product favored reaction has Keq =
Q119: Increasing the pressure will _. 2NH₃ (g)
Q120: Write an expression for the equilibrium constant
Q122: Changing the temperature of a reaction will
Q123: Predict the shift in the direction of
Q124: Predict the effect of increasing pressure in
Q125: Write an expression for the equilibrium constant
Q126: If the value of the equilibrium constant