Essay
Write an expression for the equilibrium constant (Keq) for the following reaction. 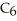
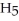
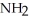 (aq) +
(aq) + 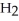 O (l)
O (l) 
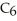
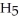
 + (aq) + OH- (aq)
+ (aq) + OH- (aq)
Correct Answer:

Verified
Keq = [ 
 ...
...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Keq = [ 
 ...
...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q120: Write an expression for the equilibrium constant
Q121: Write an expression for the equilibrium constant
Q122: Changing the temperature of a reaction will
Q123: Predict the shift in the direction of
Q124: Predict the effect of increasing pressure in
Q126: If the value of the equilibrium constant
Q127: If the value of the equilibrium constant
Q128: Predict the effect of increasing the concentration
Q129: Among the two reactions whose equilibrium constants
Q130: At 500 K, the equilibrium concentrations of