Multiple Choice
Predict the effect of increasing pressure in each of the reactions by choosing the appropriate direction shift.
-2 C?H?? (g) + 25 O? (g) 
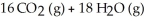
A) no effect
B) right (?)
C) left (?)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q119: Increasing the pressure will _. 2NH₃ (g)
Q120: Write an expression for the equilibrium constant
Q121: Write an expression for the equilibrium constant
Q122: Changing the temperature of a reaction will
Q123: Predict the shift in the direction of
Q125: Write an expression for the equilibrium constant
Q126: If the value of the equilibrium constant
Q127: If the value of the equilibrium constant
Q128: Predict the effect of increasing the concentration
Q129: Among the two reactions whose equilibrium constants