Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of O2. 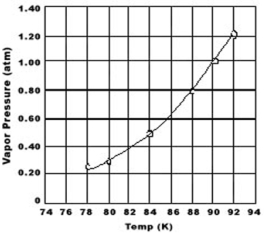
A) 92 K
B) 90.K
C) 88 K
D) 84 K
E) 78 K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: _ is the name given to the
Q26: The strongest intermolecular interactions between hydrogen fluoride
Q27: Which one of the following crystallizes in
Q80: Which substance has the highest vapor pressure
Q89: Vanadium crystallizes in a body-centered cubic lattice,
Q109: Which one of the following involves ion-dipole
Q115: The vapor pressure of ethanol is 400.
Q125: What is defined as the number of
Q129: Consider the phase diagram shown below. <img
Q134: Which has the highest surface tension at