Exam 15: Infrared Spectroscopy and Mass Spectrometry
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties86 Questions
Exam 2: Molecular Representations114 Questions
Exam 3: Acids and Bases72 Questions
Exam 4: Alkanes and Cycloalkanes66 Questions
Exam 5: Stereoisomerism94 Questions
Exam 6: Chemical Reactivity and Mechanisms86 Questions
Exam 7: Substitution Reactions103 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions76 Questions
Exam 9: Addition Reactions of Alkenes40 Questions
Exam 10: Alkynes161 Questions
Exam 11: Radical Reactions52 Questions
Exam 12: Synthesis55 Questions
Exam 13: Alcohols and Phenols71 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides102 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy90 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry108 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions45 Questions
Exam 18: Aromatic Compounds79 Questions
Exam 19: Aromatic Substitution Reactions64 Questions
Exam 20: Aldehydes and Ketones97 Questions
Exam 21: Carboxylic Acids and Their Derivatives58 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates89 Questions
Exam 23: Amines65 Questions
Exam 24: Carbohydrates101 Questions
Exam 25: Amino Acids, Peptides, and Proteins99 Questions
Exam 26: Lipids82 Questions
Exam 27: Synthetic Polymers85 Questions
Select questions type
Which one of the following compounds is consistent with the following IR spectrum? 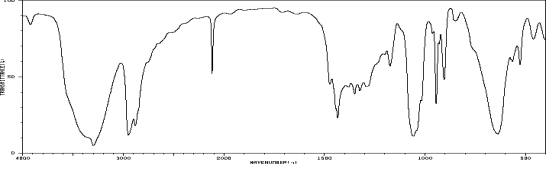 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 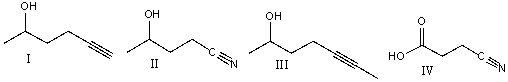
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following information is primarily obtained from infrared spectroscopy?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is currently used to indicate the location of a signal on an IR spectrum?
(Multiple Choice)
4.8/5  (32)
(32)
Which one of the following compounds will have the lowest wavenumber for carbonyl absorption? 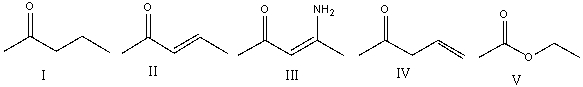
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following is expected to be the base peak in the mass spectrum of CH3(CH2)4OH?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following compounds will have a base peak at m/z=43?
(Multiple Choice)
4.9/5  (31)
(31)
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 108,relative height=61.5%
(M+1)+ . at m/z= 109,relative height=1.5%
(M+2)+ . at m/z= 110,relative height=61.3%
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is true about the (M+1)+ . peak on the mass spectrum of a hydrocarbon?
(Multiple Choice)
4.7/5  (31)
(31)
Propose possible structure(s)for a compound with molecular formula C4H7N that shows absorption at 2250 cm-1 in its IR spectrum.
(Essay)
4.9/5  (33)
(33)
Which one of the following compounds is consistent with the following IR spectrum? 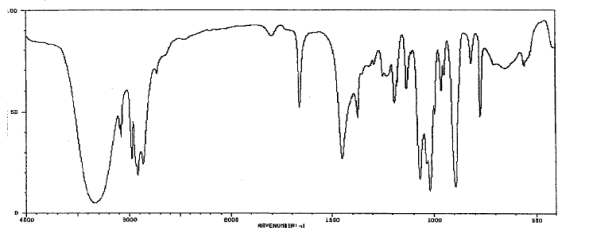 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 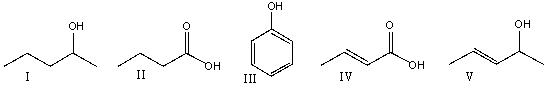
(Multiple Choice)
4.9/5  (32)
(32)
Carboxylic acids show a very broad absorption for the OH compared to alcohols,because they can form a ______.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is expected to be the base peak in the mass spectrum of 2-methyl-2-butanol?
(Multiple Choice)
4.7/5  (38)
(38)
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 84,relative height=10.0%
(M+1)+ . at m/z= 85,relative height=0.56%
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following compounds is consistent with the following IR spectrum? 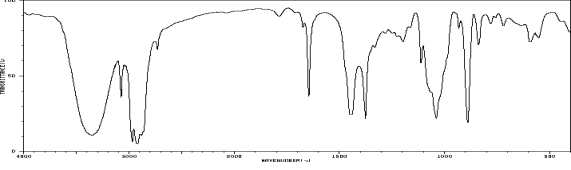 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 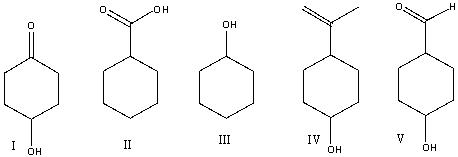
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following is true about the IR spectrum of the following compound? 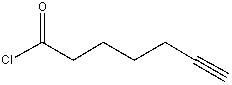
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following compounds will have the lowest wavenumber for carbonyl absorption? 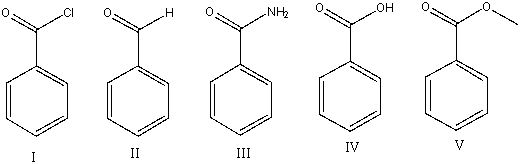
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following corresponds to the functional group region on an IR spectrum?
(Multiple Choice)
4.8/5  (45)
(45)
A compound with molecular formula C6H10O,shows absorptions at 1720 cm-1 and at 2980 cm-1 on the IR spectrum.Propose a possible structure for this compound.
(Essay)
4.7/5  (37)
(37)
Showing 21 - 40 of 108
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)