Exam 11: Radical Reactions
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties86 Questions
Exam 2: Molecular Representations114 Questions
Exam 3: Acids and Bases72 Questions
Exam 4: Alkanes and Cycloalkanes66 Questions
Exam 5: Stereoisomerism94 Questions
Exam 6: Chemical Reactivity and Mechanisms86 Questions
Exam 7: Substitution Reactions103 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions76 Questions
Exam 9: Addition Reactions of Alkenes40 Questions
Exam 10: Alkynes161 Questions
Exam 11: Radical Reactions52 Questions
Exam 12: Synthesis55 Questions
Exam 13: Alcohols and Phenols71 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides102 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy90 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry108 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions45 Questions
Exam 18: Aromatic Compounds79 Questions
Exam 19: Aromatic Substitution Reactions64 Questions
Exam 20: Aldehydes and Ketones97 Questions
Exam 21: Carboxylic Acids and Their Derivatives58 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates89 Questions
Exam 23: Amines65 Questions
Exam 24: Carbohydrates101 Questions
Exam 25: Amino Acids, Peptides, and Proteins99 Questions
Exam 26: Lipids82 Questions
Exam 27: Synthetic Polymers85 Questions
Select questions type
Free radical chlorination of ethane can produce higher halogenation products (dichlorinated,trichlorinated,etc…)in addition to chloroethane.How could the production of higher halogenated products be minimized?
(Multiple Choice)
4.9/5  (47)
(47)
Compound A has molecular formula C9H20.Compound A produces three constitutional isomers upon chlorination,and one constitutional isomer upon bromination.Which of the following are possible structures of Compound A? 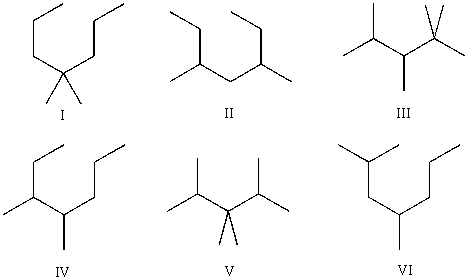
(Essay)
4.8/5  (33)
(33)
Thermal cracking of butane can produce ethyl radicals via homolytic cleavage.Use correct arrow formalism to show this process.
(Essay)
4.7/5  (36)
(36)
Compound A (C6H12)reacts with HBr/ROOR to give one compound (compound B,C6H13Br).Compound C (C6H14)reacts with bromine and light to produce one compound (compound B,C6H13Br).Suggest structures for compounds A,B,and C.
(Essay)
4.8/5  (29)
(29)
Which of the following shows the initiation step of monochlorination of methane? 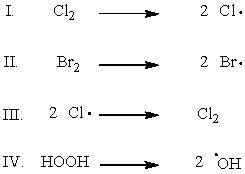
(Multiple Choice)
4.9/5  (35)
(35)
What term most accurately describes the process shown below? 
(Multiple Choice)
4.9/5  (44)
(44)
Azobisisobutyronitrile (AIBN)is commonly used as a radical initiator.Use correct arrow formalism to show this process. 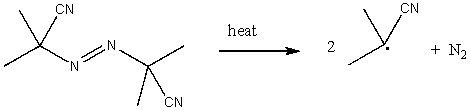
(Essay)
4.7/5  (34)
(34)
Which of the following processes is responsible for the fact that free radical bromination of methane is slower than free radical chlorination?
(Multiple Choice)
4.8/5  (38)
(38)
Use correct arrow formalism to show the propagation steps in the chlorination of propane to produce 2-chloropropane.
(Essay)
4.8/5  (40)
(40)
One possible product of thermal cracking of hexane is 1-butene.Use correct arrow formalism to suggest a possible mechanism for this process starting from an alkyl radical.
(Essay)
4.8/5  (32)
(32)
Upon treatment with NBS and irradiation with UV light,1-ethyl-4-methyl produces three monobrominated compounds (including stereoisomers).Draw the products of this reaction.
(Essay)
4.7/5  (34)
(34)
The free radical polymerization of styrene with benzoyl peroxide yields a polymer that has repeat units arranged primarily in a 'head-to-tail' arrangement.This means that the phenyl group primarily ends up placed at alternating carbon atoms along the chain.Use correct arrow formalism to show why this arrangement is preferred over a 'head-to-head' or 'tail-to-tail' arrangement. 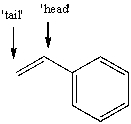
(Essay)
4.7/5  (41)
(41)
How many constitutional isomers are possible if propane is dichlorinated? Draw them.
(Essay)
4.7/5  (36)
(36)
A bromine radical can add to the pi bond of 2-methylpropene.Use correct arrow formalism to show this process and the expected result.
(Essay)
4.8/5  (37)
(37)
Showing 21 - 40 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)