Exam 1: Structure and Bonding
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds; Acids and Bases41 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry29 Questions
Exam 5: Stereochemistry at Tetrahedral Centers40 Questions
Exam 6: An Overview of Organic Reactions39 Questions
Exam 7: Alkenes and Alkynes36 Questions
Exam 8: Reactions of Alkenes and Alkynes38 Questions
Exam 9: Aromatic Compounds37 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy42 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy43 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy41 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations43 Questions
Exam 13: Alcohols, Phenols, and Thiols; Ethers and Sulfides38 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions36 Questions
Exam 15: Carboxylic Acids and Nitriles36 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions46 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions46 Questions
Exam 18: Amines and Heterocycles36 Questions
Exam 19: Biomolecules: Amino Acids, Peptides, and Proteins52 Questions
Exam 20: Amino Acid Metabolism32 Questions
Exam 21: Biomolecules: Carbohydrates49 Questions
Exam 22: Carbohydrate Metabolism45 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism42 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism34 Questions
Exam 26: Orbitals and Organic Chemistry: Pericyclic Reactionse44 Questions
Exam 27: Synthetic Polymerse35 Questions
Select questions type
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
-Which of the following statements is not true according to molecular orbital (MO) theory?
(Multiple Choice)
4.8/5  (30)
(30)
How many nonbonding electron pairs are in the structure shown below? 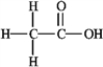
(Multiple Choice)
4.9/5  (41)
(41)
How many electrons are there in the valence shell of the carbon atom of a methyl anion, CH3−?
(Multiple Choice)
4.7/5  (39)
(39)
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
-What is the expected hybridization around the sulfur atom in diethyl sulfide? CH3CH2⎯S⎯CH2CH3
(Multiple Choice)
4.8/5  (31)
(31)
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
-In the two structures shown below, what do the positions labeled with the arrow have in common? 
(Multiple Choice)
4.8/5  (31)
(31)
Determine the hybridization for the indicated atoms in each structure below. 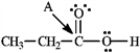
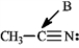 -Refer to instructions. The hybridization of carbon atom B is _____.
-Refer to instructions. The hybridization of carbon atom B is _____.
(Short Answer)
4.7/5  (42)
(42)
Draw a picture showing the orbitals involved in the π-bonds of cyclopenta-1,3-diene, a commonly encountered reagent in organic synthesis. 
(Essay)
4.8/5  (25)
(25)
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
-Refer to instructions. Which of the following correctly describes the structure of these compounds?
(Multiple Choice)
4.7/5  (45)
(45)
Showing 21 - 29 of 29
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)