Exam 13: Atmospheric Science, Air Quality, and Pollution Control
Define atmospheric deposition in general and explain the causes and effects of acidic deposition.
Atmospheric deposition refers to the wet or dry deposition of a wide variety of pollutants, including mercury, nitrates, organochlorines, and many others. Acidic deposition refers to the deposition of acidic or acid-forming pollutants. This can take place either by precipitation (primarily acid rain, but also including acid snow, sleet, and hail), by fog, by gases, or by the deposition of dry particles. Acidic deposition originates primarily with sulfur dioxide and nitrogen oxides, pollutants produced largely through fossil fuel combustion by automobiles, electric utilities, and industrial facilities. Once emitted into the troposphere, these pollutants can react with water, oxygen, and oxidants to produce compounds of low pH. Droplets of these acids may travel hundreds or thousands of kilometers. Acidic deposition can have wide-ranging, cumulative detrimental effects on ecosystems and on our built environment. It leaches basic minerals such as calcium and magnesium from the soil, changing soil chemistry and harming plants and soil organisms. Streams, rivers, and lakes may become significantly acidified from runoff. Some forests in eastern North America have experienced widespread tree dieback from these conditions. Acidic precipitation also may damage stone buildings, eat away at cars, and erase the writing from tombstones.
The Coriolis effect contributes to _________________
B
If all of the stratospheric ozone suddenly disappeared,_________________ .
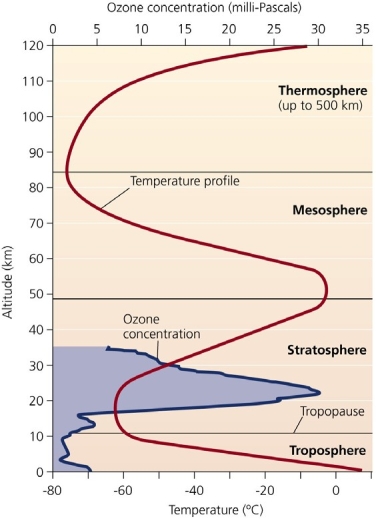 Use the figure above to answer the following question
-At least for the stratosphere,______________ .
Use the figure above to answer the following question
-At least for the stratosphere,______________ .
Which of the following is a consequence of acidic deposition?
The largest portion of atmospheric gases is _________________.
Water soluble sulfur and nitrogen oxides produced by burning fossil fuels can be removed from an industrial source by _________________.
You have been hired by a rapidly growing small city to improve the air quality, which has deteriorated in the past 10 years. Your first suggestion is to _________________
When coal or oil are burned, some part of it is completely combusted, forming CO2; another part is partially combusted, forming CO (carbon monoxide); and some remains unburned and is released as soot. Along with sulfur dioxide, these are the main components of _________________.
Read the following scenario and answer the question below.
Thousands of young families moved ʺover the hillsʺ and into the San Fernando Valley, a suburb of Los Angeles, after World War II. New neighborhoods were springing up, replacing orange groves and open space; roads and schools quickly sprang into existence, trying to keep pace with the rapid population growth. Ringed by beautiful mountains, the entire Los Angeles basin looked like a new, green, sun-filled paradise to the families seeking a fresh start. In the early 1950s, one of the common family chores in Los Angeles was to carry the trash out to the stone incinerator behind the garage where each family burned all of their dry trash. ʺWetʺ garbage was collected and taken to a city dump, where it was burned by the city. Everyone throughout the city either used an incinerator or burned things in an open trash pile; there were over 400,000 backyard trash incinerators. On warm afternoons, eyes would sometimes sting and burn. People would stop, close their eyes, and let the cleansing tears refresh irritated eyes. They accepted this as a normal part of life in sunny California.
-In the mid-1950s, a researcher in Los Angeles was able to create smog by setting up a large, clear chamber and using it to expose auto exhaust to sunlight. He was demonstrating _________________
Bacteria and fungal spores can be included as _________________
One of the problems that occur as a consequence of CFC pollution is _________________
In developed countries, the two most deadly sources of indoor pollution are _________________
Photochemical smog differs from industrial smog in that it _________________
The primary causes of acid deposition are _________________
Ozone in the _________________ is important for absorbing and scattering much of the UV radiation coming into Earthʹs atmosphere from the sun.
Why is indoor pollution still such a large problem? What are the most dangerous indoor pollutants in developing and developed nations?
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)