Exam 13: The Solid State
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
Nickel crystallizes in a face-centered cubic lattice. The density of the nickel is 8.91 g/cm3. What is the volume of a single unit cell?
(Multiple Choice)
4.7/5  (25)
(25)
The lattice energy of NaBr is -752 kJ/mol. This energy corresponds to which of the following reactions?
(Multiple Choice)
4.8/5  (40)
(40)
Cesium bromide crystallizes in a primitive cubic unit cell with bromide ions at the lattice points. The cesium ions occupy cubic holes. How many bromide ions surround each cesium ion in cesium bromide?
(Multiple Choice)
4.9/5  (38)
(38)
If a metal crystallizes in a body-centered cubic lattice, each metal atom has _____ "nearest neighbors."
(Multiple Choice)
4.8/5  (34)
(34)
Sodium chloride crystallizes in a(n) _____ cubic unit cell with chloride ions occupying the lattice points. The sodium ions occupy interstitial regions, with each cation in contact with six chloride ions.
(Short Answer)
4.7/5  (39)
(39)
A salt with a 1:1 ratio of anions to cations may pack in a face-centered cubic unit cell with the anions at the lattice points and the cations occupying one-half of the _____ holes. Zinc sulfide is an example of this structure.
(Short Answer)
4.8/5  (33)
(33)
Which of the following ionic compounds is expected to have the lowest enthalpy of fusion?
(Multiple Choice)
4.9/5  (32)
(32)
Copper crystallizes in a face-centered cubic lattice. The radius of a copper atom is 128 pm. What is the edge length of the unit cell?
(Multiple Choice)
4.8/5  (37)
(37)
Lithium chloride crystallizes in a face-centered cubic unit cell with chloride ions occupying the lattice points and lithium ions occupying octahedral holes. How many chloride ions surround each lithium ion in LiCl?
(Multiple Choice)
4.8/5  (41)
(41)
The energy of formation of one mole of solid crystalline ionic compound when ions in the gas phase combine is referred to as _____.
(Multiple Choice)
4.9/5  (35)
(35)
Magnesium sulfide (molar mass 56.37 g/mol) has a face-centered cubic unit cell with magnesium ions in octahedral holes. The ionic radii of magnesium ions and sulfide ions are 79 pm and 184 pm, respectively. What is the density of MgS (in g/cm3)?
(Multiple Choice)
5.0/5  (40)
(40)
Which two of the following materials are most likely to be amorphous solids: ice, nylon, glass, potassium nitrate?
(Multiple Choice)
4.9/5  (35)
(35)
If an ionic compound with the formula MX forms a primitive cubic unit cell with the anions (Xn-) at the lattice points, the cations (M2n+) will occupy:
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following substances is incorrectly matched with the kind of solid it forms? Substance/Kind of Solid
(Multiple Choice)
4.9/5  (45)
(45)
The metal sodium crystallizes in a body-centered cubic lattice. If the density of sodium is 0.968 g/cm3, what is the unit cell edge length?
(Multiple Choice)
4.9/5  (39)
(39)
Which process requires the greatest endothermic change in enthalpy for water?
(Multiple Choice)
4.9/5  (39)
(39)
A low-melting solid readily dissolves in water to produce a nonconducting solution. The solid is most likely a(n) _____.
(Multiple Choice)
4.9/5  (43)
(43)
Using the following data to calculate the lattice energy of NaBr(s). ?IE and ?EA are enthalpy of ionization and electron attachment enthalpy, respectively. ()\rightarrow() =+107 ()\rightarrow()+ \DeltaIE=+496 1/2()\rightarrow() =+112 ()+\rightarrow() \DeltaEA=-325 ()+1/2()\rightarrow() =-361
(Multiple Choice)
4.9/5  (44)
(44)
On the phase diagram below, which point corresponds to conditions where only the gas phase exists? 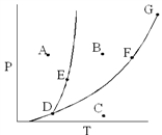
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 60 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)