Exam 13: The Solid State
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
Above a substance's _____ temperature, it is not possible to compress the substance into the liquid phase. If enough pressure is applied the substance will become a supercritical fluid.
(Short Answer)
4.9/5  (37)
(37)
If an ionic solid has a face-centered cubic lattice of anions (Xn−) and all the octahedral holes are occupied by metal cations (Mm+), what is the formula for the compound?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following elements might be used as a dopant in a silicon host to create a p-type semiconductor?
(Multiple Choice)
4.7/5  (40)
(40)
Nickel has a face-centered cubic cell, and its density is 8.90 g/cm3. What is the radius (in pm) of a nickel atom? (The molar mass of nickel is 58.69 g/mol)
(Multiple Choice)
4.8/5  (27)
(27)
According to the below phase diagram, what process occurs if a pure substance begins at point C and the pressure on the substance is increased until point B is reached? 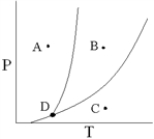
(Multiple Choice)
4.7/5  (40)
(40)
A metal crystallizes in a face-centered cubic lattice. The atomic radius of the metal is 198 pm and the density of the metal is 6.57 g/cm3. What is the volume of the unit cell?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following statements is true about semiconductors?
(Multiple Choice)
4.9/5  (32)
(32)
If an ionic compound with the formula MX2 forms a face-centered cubic unit cell with the cations (M2n+) at the lattice points, the anions (Xn-) will occupy:
(Multiple Choice)
4.8/5  (33)
(33)
What is the simplest formula of the compound represented by the unit cell provided below? 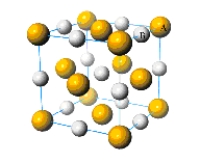
(Multiple Choice)
4.8/5  (31)
(31)
In metals, there are not enough electrons to fill all of the electronic energy levels. At 0 K, the highest energy level filled is referred to as the ________.
(Multiple Choice)
5.0/5  (29)
(29)
Point D on the phase diagram is referred to as the _____ point. 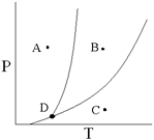
(Multiple Choice)
4.9/5  (36)
(36)
What is the length of the diagonal (in atomic radii) along the face of a face-centered cubic unit cell?
(Multiple Choice)
4.8/5  (37)
(37)
Strontium oxide has a face centered cubic unit cell of oxide ions with the strontium ions in octahedral holes. If the radius of Sr2+ is 127 pm and the radius of O2- is 135 pm, what is the density of strontium ions oxide? (100 cm = 1 × 1012 pm)
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following compounds is expected to have the strongest ionic bonds?
(Multiple Choice)
4.9/5  (43)
(43)
Cesium crystallizes in the body-centered cubic system. If the edge of the unit cell is 612 pm, what is the radius of a cesium atom in picometers?
(Multiple Choice)
4.9/5  (44)
(44)
Iron(II) sulfide has a primitive cubic unit cell with sulfide ions at the lattice points. The ionic radii of iron(II) ions and sulfide ions are 88 pm and 184 pm, respectively. What is the density of FeS (in g/cm3)?
(Multiple Choice)
4.8/5  (38)
(38)
Palladium crystallizes in a face-centered cubic unit cell. If the edge length of the unit cell is 387 pm, what is the radius of a palladium atom in picometers?
(Multiple Choice)
4.8/5  (32)
(32)
Polonium (atomic mass 209.0 g/mol) crystallizes in a primitive cubic unit cell. If the density of polonium is 9.15 g/cm3, what is the radius of a polonium atom (in pm)?
(Multiple Choice)
4.9/5  (31)
(31)
Iron crystallizes in a body-centered cubic lattice. If the radius of iron is 126 pm, what is the unit cell edge length?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 21 - 40 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)